Have a specific question about your LBP project? Click below and let’s get started.
Recombinant Protein Production
List Labs has decades of experience working with recombinant protein production for R&D and Phase I and II clinical trials.


List Labs has decades of experience working with recombinant protein production for R&D and Phase I and II clinical trials.

Recombinant proteins have achieved profound success in the biotechnology and pharmaceutical industries, and have been used in the production of vaccines and therapeutics to treat a wide variety of conditions. Protein expression in Escherichia coli and Bacillus subtilis have been the most widely utilized means of producing recombinant proteins and enzymes. E. coli and B. subtilis are well-understood host organisms that offer straightforward genetic modification and a rapid growth profile.
List Labs has a long history of manufacturing recombinant proteins of bacterial or mammalian origin to support our clients’ projects from Research and Development through Phase I and Phase II clinical trials. With the protein of interest in a suitable expression system, the process is optimized and can be scaled up to facilitate large scale production of up to 100L, or 500L of culture. Target proteins are isolated from the cell lysate soluble fractions or from inclusion bodies to yield high purity recombinant proteins that can be further processed to remove affinity tags per client request prior to product packaging.
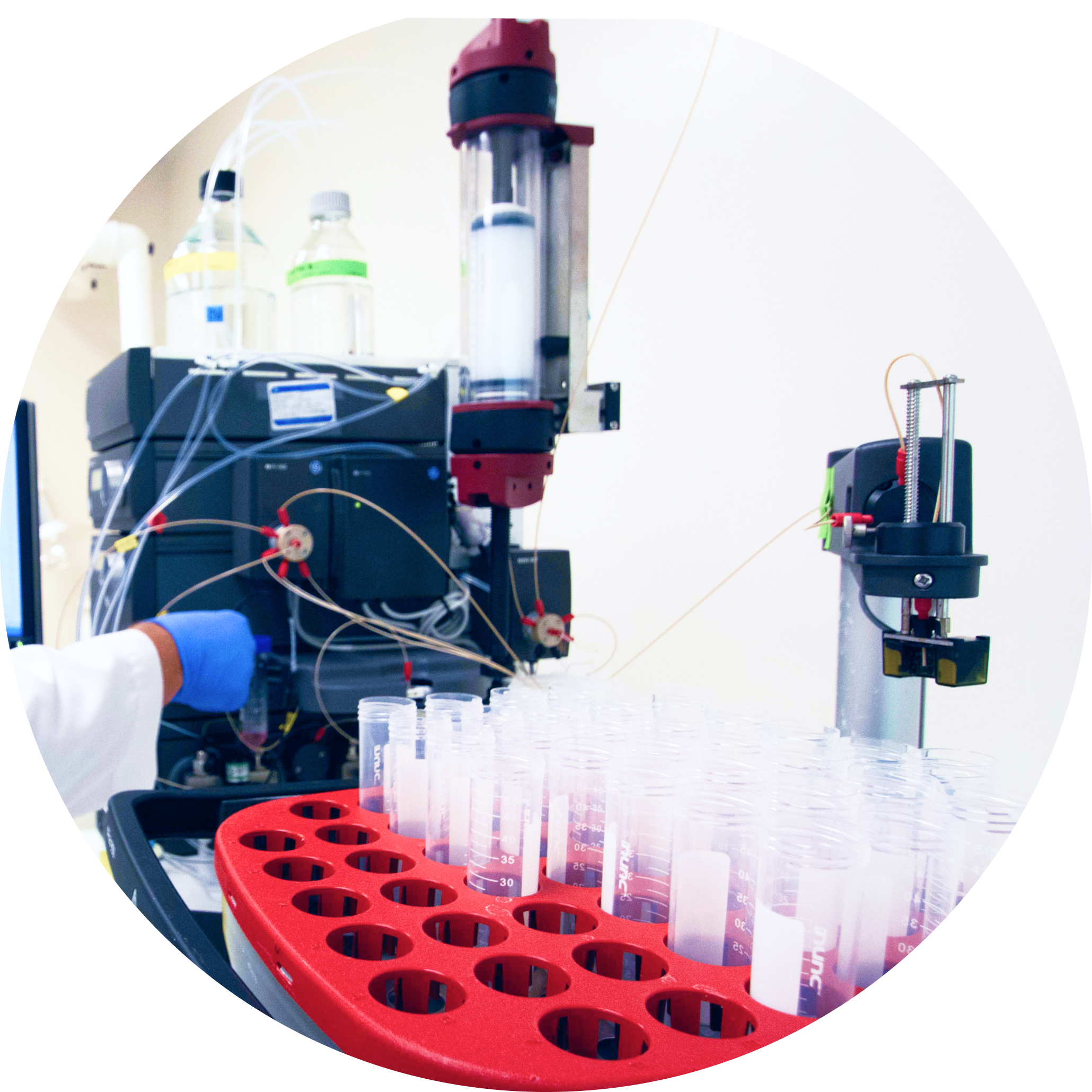
List Labs has the experience and agility to meet the unique needs of your recombinant protein projects. We establish a reproducible and robust process with the following development services.
Following an initial tech transfer — to acquire familiarity with the recombinant protein expression system and replicate client data and results — small scale work is designed to support a seamless transition to a process that will support large-scale cGMP manufacturing.
To support process development and future process validation, clients are recommended to provide in-process tests to aid in the evaluation of each step’s success, as well as to assess the final product.
Leveraging our considerable experience with suitability testing, we tailor analytical method development to the desired product as well as generally applicable analysis described in the USP. These tests may include product activity, antigenicity, endotoxin content, moisture, water activity, and osmolality.

List Labs’ highly skilled team has a wealth of experience with cGMP manufacturing of recombinant protein products from E. coli, B. subtilis and many other strains . Our expertly designed manufacturing facility provides containment in ISO7 or ISO8 suites, and multiple production suites allowing parallel cGMP manufacturing activities to streamline project timelines.
List Labs has several large-scale options for the production of recombinant proteins including 100L stainless steel vessels with validated cleaning procedures, and a 500L Single Use bioreactor.
Downstream production capabilities include:
Have a specific question about your LBP project? Click below and let’s get started.
List Labs
540 Division Street
Campbell, California
List Biotherapeutics
9800 Crosspoint BIvd Suite 2011
Indianapolis, IN 46256, USA


