Have a specific question about your LBP project? Click below and let’s get started.
I’d like to tell you about some of the obstacles you may encounter as you develop your live biotherapeutic product (LBP) and how List Labs can help you navigate through them.
Harnessing bacteria’s potential for a healthier world is our company mission. Our history and experience have been devoted to bacteria – what bacteria can produce – cultivating bacteria, purifying proteins, and polysaccharides. List Lab’s passion is to support innovators with quality bacterial products for research and development of vaccines and medical products and to perform contract development and manufacturing service for transformative therapies such as LBPs.
I am very excited to share that List Labs has partnered with BetterLife Pharma to develop and manufacture Altum Pharmaceutical’s novel and transformative therapeutic for the treatment of COVID-19. It is an honor to join in the fight against one of the greatest challenges of this decade.
Who is List Labs?
List is a privately held, woman-owned, and operated company in California, and about a quarter of our staff has advanced degrees. Initially, the core part of our business was manufacturing bacterial products. Beginning with selling one bacterial product in 1978, we now have over 100 stock products, including a GMP product. List Labs is a GMP-compliant facility, but we’re more than a collection of state-of-the-art equipment, List is much greater than the sum of its parts. Live biotherapeutic projects are not a cookie-cutter process and we are uniquely qualified to provide insight and flexibility to match the needs and requirements of each individual project. We typically take on 2 to 3 microbiome projects at a time, providing your project with individual attention and the critical advantage necessary to achieve a successful outcome.
Our cGMP compliant facility has 7 expertly designed manufacturing suites allowing segregation of product campaigns and spore containment if needed. The suites undergo treatment with vaporous hydrogen peroxide prior to GMP manufacturing to ensure the quality of your product. Due to the design of the facility and equipment options, we have the ability to manufacture several products in parallel.
Leveraging decades of experience cultivating a variety of microorganisms and GMP manufacturing experience for our products and partners, the transition to the microbiome space was a natural extension of our capabilities and expertise. We began a project about 7 years ago with a partner to develop and manufacture a live bacteria product. One of the first in the burgeoning microbiome field.
We have now worked on dozens of projects for indications in the gut, skin, women’s vaginal and urinary tract health, and CNS. We manufacture products for Phase I and II clinical trials. And we have produced over 20 different LBP products. These projects not only include manufacturing but also typically require a lot of development, many of which come straight from the academic bench with very little development past a shake flask or bottle cultivation. We are a partner who is invested in the success of your project and work as an extension of your team.
Let’s use climbing Mt Everest as an analogy. We are climbing the mountain with you, right alongside you. It’s a great analogy because it gets harder and harder the higher you get, and what you do at the bottom, your preparation will make or break you at the top. Aggressive timelines, budget constraints, these are the shear cliffs we can see but so many of the obstacles that await are unseen black ice, or bottomless crevasse’s… I know it’s a bit dramatic but getting a LBP to market is much the same, full of potential pitfalls.
So, today I want to share with you what some of these pitfalls look like and give you a glimpse of how List Labs can help you avoid becoming one of the many, that never realize the ultimate goal.
Source of Strain
If there is anything you take home from this talk, it should be this. “Choose Wisely!” Your choice of strain is made early in the project and changing mid-ascent even to “quote” the same species has a compounding impact. If you need to change strains, all of your in vivo and in vitro assay will need to be repeated along with all your development. The result can be very costly and cause a substantial delay to your timeline. A strain identified as a particular species is not equal to another strain identified as the same species. Each is unique and the characteristics or phenotypes that are important for your strain and indication may not be representative of another strain although identified as the same species.
But whatever your strain of choice is, it is highly likely that we have worked with it or a similar strain before. This is a sample list, although not exhaustive, of the strains we have worked with and of course not giving away any confidentiality of our clients.
Animal-Free Media Replacement
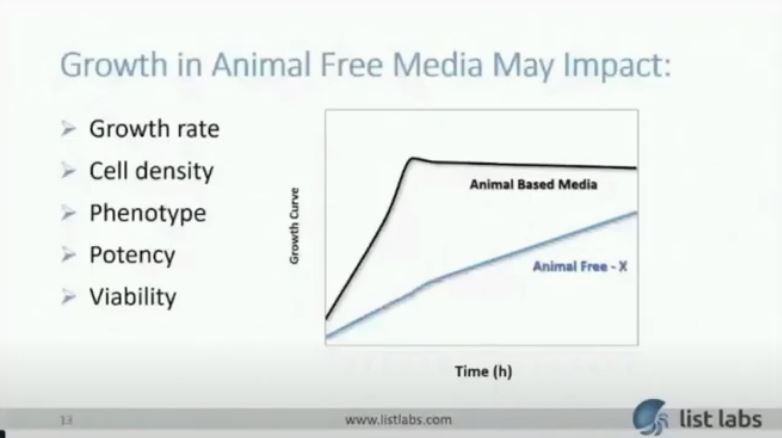
Another potential obstacle is replacing the media with animal-free alternatives. Since you need to establish a product that is BSE/TSE-free, many clients choose to switch to animal-free media. This is not trivial as there are many animal-derived components that are difficult to replace and are necessary for the robust growth of the organism. Shown in the graph below, is an animal-based media compared to an animal-free base media missing a critical animal-derived component which when the client came to us they had been unsuccessful in replacing. Growth in an animal-free media may impact growth rate, final cell density, phenotype (which may be an important characteristic for your indication), potency, and viability. All of which has implications to the scale of the process in order to have enough viable cells for your dose requirements. This directly impacts your Return on investment.
But we know how to tackle the problem. This is an example using the graph I showed you before where growth rate and final cell density were impacted without a very critical animal-derived component. Once we identified a suitable replacement, we demonstrated similar or better growth than with the animal-derived media. Then we realized further improvements in cell density with process improvements resulting in a 5-10X improvement in cell density.
Preserving Viability
Another pitfall is loss in viability of your organism through the process. This is a typical manufacturing process flow for a live biotherapeutic product. Initially, the strain is cultivated in a seed culture either anaerobically or aerobically and inoculated into the large vessel (such as a stainless steel bioreactor or single-use bioreactor), then harvested by tangential flow filtration, formulated, lyophilized, sieved, and then filled into vials, applicators, or formulated to fill capsules.
Different organisms will have different sensitivities to the process that may impact its viability including the growth phase at which the strain was harvested, the harvest process, and the environment during the harvest, or during the lyophilization, sieving, and encapsulation process. Understanding the viability of the organism throughout the process is important to know where to focus your development efforts.
We understand these risks and what tools we can use to improve the yield of the live organism at the end of your process. We have demonstrated substantial improvements in viability by 2 to 100 fold by optimizing these variables.
Scale Up
Another hurdle to overcome is the scale-up of the process. We often start with a process that is at the tube, bottle, or shake flask scale which requires a substantial amount of scale-up development. The process development of the cultivation and harvest should be performed in a scaled-down version of the process such that performance will be predictable at scale with the necessary process controls to demonstrate similar performance. List has 1L bioreactors and scaled-down versions of the harvest process so we can isolate specific variables and understand the impact on the process. We typically perform 10X scale up from 1L to 10L to the 100L process to minimize the surprises at scale and provide a robust process. Your timeline and costs for development should include these activities.
QC Analytical Development
An obstacle of the QC Analytical Development of your LBP is the development of the bioburden assays, USP<61>/<62> which is not straightforward with a live organism. For LBPs these assays need to be tailored and developed for each organism and we have experience working on these assays. We are also able to harness a lot of efficiencies for the customer by not only working on the manufacturing but also working on the analytical development in-house. Analytical assays are necessary for both in-process testing and final QC testing for release.
Is List Labs the right CDMO for your project?
As a novice climber, would you attempt to scale Mt. Everest with anything less than the most experienced Sherpa? We are a passionate and dedicated team with the expertise and experience that is critical to reach the summit. We have been there many times. We recognize the obstacles and while no two ascents are exactly the same, we have the flexibility to guide you to the top and deliver a quality product for the success of your project. We look forward to working with you!
If you have any questions, please contact us at services@listlabs.com or through our contact page.
By: Stacy Burns-Guydish, Ph.D., President
Check out the Overcoming Obstacles in the development of Live Biotherapeutic Products video!