Have a specific question about your LBP project? Click below and let’s get started.
6 Common Pitfalls in CDMO Partnerships—and How to Avoid Them
Intro
From small biotech startups to global pharmaceutical giants, many companies rely on Contract Development and Manufacturing Organizations (CDMOs) to bring their products to market more efficiently. Outsourcing can accelerate timelines and reduce up-front investment in facilities, but it also brings risks if not managed properly. Below are six common pitfalls teams face when partnering with a CDMO—and strategies to ensure a smoother, more successful collaboration.
1. Overlooking Detailed Project Scope and Goals
Pitfall: In the rush to get started, sponsors often skip a thorough scope definition. They provide only high-level objectives, hoping the CDMO’s expertise will “fill in the gaps.”
Solution:
- Align Early on Project Details: Outline deliverables, critical milestones, and quality criteria in a written document.
- Define Success Metrics: Whether it’s yield targets for an upstream fermentation process or specific release criteria for finished drug product, discuss the metrics with your CDMO to incorporate the necessary development required in the scope of work.
- Use a Phase-Gate Approach: Build in formal checkpoints (e.g., pre-clinical scale-up, pilot runs, GMP batch release) to review progress and adapt plans.
Why It Matters: Without clear scope and goals, small misunderstandings can balloon into missed timelines and budget overruns.
List Labs Inside Tip: Having a detailed plan makes it possible to compare CDMO partners on a level playing field. Don’t assume that the price you see encompasses all the activities that you expect it to. If a CDMO isn’t willing to go through this exercise with you to earn your business, what will it be like working with them once you have committed?
2. Underestimating CDMO Technology Transfer Complexities
Pitfall: Some sponsors assume a direct “plug-and-play” transfer of their lab protocols to the CDMO’s facilities. In reality, technology transfer can be one of the most challenging parts of the partnership.
Solution:
- Conduct Feasibility Assessments: Before large-scale work begins, perform scaled-down runs or pilot runs.
- Process Transfer: Provide detailed SOPs, process parameters, and analytical methods. Record learnings in a tech transfer package or report.
- Collaborate Closely: Assign dedicated points of contact—both for the sponsor and the CDMO—to manage the data exchange.
Why It Matters: Properly executed tech transfer saves time and money by smoothing out issues before they become major roadblocks at scale.
List Labs Inside Tip: In the development stages, what makes a process work vs. not work can be unknown. There may be critical factors that you are not even aware are critical. Differences in equipment, technique, or a myriad of other factors, may influence how the process works in our hands versus yours. We can’t emphasise the importance of this step enough.
3. Neglecting Regulatory and Compliance Requirements
Pitfall: Some sponsors forget that a CDMO must adhere not just to FDA cGMP or EMA GMP but potentially to global standards if the product will be marketed internationally. Missing a single compliance step can lead to costly delays.
Solution:
- Confirm Compliance: Ensure the CDMO has cGMP compliance according to the FDA, EMA, or PMDA and is able to host an audit to demonstrate compliance.
- Check Regulatory Support: If you need help with an IND or MAA submission, working with a regulatory consultant with vested interest in your success is highly advised.
- Stay Updated: Regulatory guidelines evolve. Establish a process for ongoing compliance checks and updates.
Why It Matters: Ensuring compliance from the get-go is far more cost-effective than dealing with product recalls or remediation efforts later.
List Labs Inside Tip: Early phase cGMP drug manufacturing sites do not require FDA certification. If an early phase CDMO has been audited by the FDA, this could be due to a past adverse event, and a red flag.
4. Assuming Infinite CDMO Capacity and Rapid Timelines
Pitfall: It’s easy to assume a CDMO always has available capacity and can meet any timeline you propose. In reality, many leading CDMOs juggle multiple projects, causing scheduling conflicts.
Solution:
- Map Out Resource Availability: During initial discussions, request a capacity forecast and typical lead times.
- Build in Buffer: If you have a tight clinical milestone (e.g., needing trial materials by Q4), pad your timeline for unexpected delays.
- Raw Material Availability: Knowing what raw materials and disposables are required for your project and researching the lead time to acquire them, can prevent unexpected delays.
Why It Matters: Realistic timelines and capacity checks help prevent last-minute surprises or bottlenecks that can derail entire development programs.
List Labs Inside Tip: Start conversations with CDMO’s sooner rather than later. You may think you are not far enough along, but those early conversations can lead to time savings down the road. For example, if there are activities in your process that are not scalable, or compatible with GMP, better to make those adjustments early.
5. Underestimating the Financial Implications of Project Changes
Pitfall: Project plans rarely stay static—especially in pharmaceutical drug product development . But each change (e.g., altering formulation, adjusting batch size) can trigger unplanned costs.
Solution:
- Establish a Change Control Process: Any modification to the scope, timeline, or technical details should be evaluated for cost and schedule impact.
- Keep a Contingency Budget: Set aside a portion (e.g., 10–15%) of the total budget for unexpected scope expansion.
Why It Matters: Clear financial oversight prevents tension between sponsor and CDMO—and helps keep your project on track fiscally.
List Labs Inside Tip: By choosing a CDMO with decades of experience, you will likely be the recipient of some sage advice that will inevitably help reduce or eliminate the need for costly changes later. We recommend heeding that advice!
6. Relying on One-Way or Sporadic Communication with Your CDMO
Pitfall: In a purely transactional relationship, the CDMO updates the sponsor only when they must (e.g., monthly reports or critical deviations). This can leave sponsors in the dark about emerging issues.
Solution:
- Regular Check-Ins: Schedule weekly or biweekly calls, plus a monthly governance meeting.
- Set Communication Protocols: Decide how quickly urgent updates (like QC failures) will be escalated and via which channels (email, phone call, project portal).
- Collaborative Tools: Use shared project management software or cloud-based data rooms to keep both teams aligned in real time.
Why It Matters: Frequent, transparent communication fosters trust and allows both parties to address potential problems before they escalate.
List Labs Inside Tip: Having a dedicated project manager as your main point of contact is vital to ensuring effective, efficient communication.
Successful CDMO Partnerships
CDMO partnerships can be a game-changer for companies looking to bring novel therapeutics—whether biologics, protein, or Live Biotherapeutic Products —to market faster and more efficiently. But success hinges on clear communication, robust planning, and mutual understanding of expectations.
By avoiding these six pitfalls—from scope definition to communication—you’ll be well on your way to a productive, trust-based relationship that benefits both parties. After all, the right CDMO isn’t just a vendor; it’s a partner in the journey to your success to deliver safe, effective treatments to patients worldwide.
Interested in More?
If you’d like additional insights into optimizing your CDMO collaboration, stay tuned for our next blog post on key metrics to track during tech transfer and how to manage regulatory audits effectively. Feel free to leave a comment or question below, and our experts will get back to you. Interested in discussing your project with us? Contact us today!
Disclaimer: The contents of this blog post are for informational purposes only and do not constitute legal or regulatory advice.
I’d like to tell you about some of the obstacles you may encounter as you develop your live biotherapeutic product (LBP) and how List Labs can help you navigate through them.
Harnessing bacteria’s potential for a healthier world is our company mission. Our history and experience have been devoted to bacteria – what bacteria can produce – cultivating bacteria, purifying proteins, and polysaccharides. List Lab’s passion is to support innovators with quality bacterial products for research and development of vaccines and medical products and to perform contract development and manufacturing service for transformative therapies such as LBPs.
I am very excited to share that List Labs has partnered with BetterLife Pharma to develop and manufacture Altum Pharmaceutical’s novel and transformative therapeutic for the treatment of COVID-19. It is an honor to join in the fight against one of the greatest challenges of this decade.
Who is List Labs?
List is a privately held, woman-owned, and operated company in California, and about a quarter of our staff has advanced degrees. Initially, the core part of our business was manufacturing bacterial products. Beginning with selling one bacterial product in 1978, we now have over 100 stock products, including a GMP product. List Labs is a GMP-compliant facility, but we’re more than a collection of state-of-the-art equipment, List is much greater than the sum of its parts. Live biotherapeutic projects are not a cookie-cutter process and we are uniquely qualified to provide insight and flexibility to match the needs and requirements of each individual project. We typically take on 2 to 3 microbiome projects at a time, providing your project with individual attention and the critical advantage necessary to achieve a successful outcome.
Our cGMP compliant facility has 7 expertly designed manufacturing suites allowing segregation of product campaigns and spore containment if needed. The suites undergo treatment with vaporous hydrogen peroxide prior to GMP manufacturing to ensure the quality of your product. Due to the design of the facility and equipment options, we have the ability to manufacture several products in parallel.
Leveraging decades of experience cultivating a variety of microorganisms and GMP manufacturing experience for our products and partners, the transition to the microbiome space was a natural extension of our capabilities and expertise. We began a project about 7 years ago with a partner to develop and manufacture a live bacteria product. One of the first in the burgeoning microbiome field.
We have now worked on dozens of projects for indications in the gut, skin, women’s vaginal and urinary tract health, and CNS. We manufacture products for Phase I and II clinical trials. And we have produced over 20 different LBP products. These projects not only include manufacturing but also typically require a lot of development, many of which come straight from the academic bench with very little development past a shake flask or bottle cultivation. We are a partner who is invested in the success of your project and work as an extension of your team.
Let’s use climbing Mt Everest as an analogy. We are climbing the mountain with you, right alongside you. It’s a great analogy because it gets harder and harder the higher you get, and what you do at the bottom, your preparation will make or break you at the top. Aggressive timelines, budget constraints, these are the shear cliffs we can see but so many of the obstacles that await are unseen black ice, or bottomless crevasse’s… I know it’s a bit dramatic but getting a LBP to market is much the same, full of potential pitfalls.
So, today I want to share with you what some of these pitfalls look like and give you a glimpse of how List Labs can help you avoid becoming one of the many, that never realize the ultimate goal.
Source of Strain
If there is anything you take home from this talk, it should be this. “Choose Wisely!” Your choice of strain is made early in the project and changing mid-ascent even to “quote” the same species has a compounding impact. If you need to change strains, all of your in vivo and in vitro assay will need to be repeated along with all your development. The result can be very costly and cause a substantial delay to your timeline. A strain identified as a particular species is not equal to another strain identified as the same species. Each is unique and the characteristics or phenotypes that are important for your strain and indication may not be representative of another strain although identified as the same species.
But whatever your strain of choice is, it is highly likely that we have worked with it or a similar strain before. This is a sample list, although not exhaustive, of the strains we have worked with and of course not giving away any confidentiality of our clients.
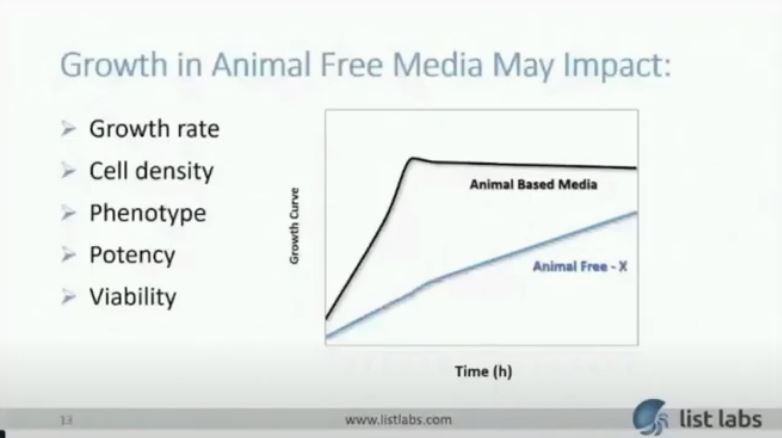
Animal-Free Media Replacement
Another potential obstacle is replacing the media with animal-free alternatives. Since you need to establish a product that is BSE/TSE-free, many clients choose to switch to animal-free media. This is not trivial as there are many animal-derived components that are difficult to replace and are necessary for the robust growth of the organism. Shown in the graph below, is an animal-based media compared to an animal-free base media missing a critical animal-derived component which when the client came to us they had been unsuccessful in replacing. Growth in an animal-free media may impact growth rate, final cell density, phenotype (which may be an important characteristic for your indication), potency, and viability. All of which has implications to the scale of the process in order to have enough viable cells for your dose requirements. This directly impacts your Return on investment.
But we know how to tackle the problem. This is an example using the graph I showed you before where growth rate and final cell density were impacted without a very critical animal-derived component. Once we identified a suitable replacement, we demonstrated similar or better growth than with the animal-derived media. Then we realized further improvements in cell density with process improvements resulting in a 5-10X improvement in cell density.
Preserving Viability
Another pitfall is loss in viability of your organism through the process. This is a typical manufacturing process flow for a live biotherapeutic product. Initially, the strain is cultivated in a seed culture either anaerobically or aerobically and inoculated into the large vessel (such as a stainless steel bioreactor or single-use bioreactor), then harvested by tangential flow filtration, formulated, lyophilized, sieved, and then filled into vials, applicators, or formulated to fill capsules.
Different organisms will have different sensitivities to the process that may impact its viability including the growth phase at which the strain was harvested, the harvest process, and the environment during the harvest, or during the lyophilization, sieving, and encapsulation process. Understanding the viability of the organism throughout the process is important to know where to focus your development efforts.
We understand these risks and what tools we can use to improve the yield of the live organism at the end of your process. We have demonstrated substantial improvements in viability by 2 to 100 fold by optimizing these variables.
Scale Up
Another hurdle to overcome is the scale-up of the process. We often start with a process that is at the tube, bottle, or shake flask scale which requires a substantial amount of scale-up development. The process development of the cultivation and harvest should be performed in a scaled-down version of the process such that performance will be predictable at scale with the necessary process controls to demonstrate similar performance. List has 1L bioreactors and scaled-down versions of the harvest process so we can isolate specific variables and understand the impact on the process. We typically perform 10X scale up from 1L to 10L to the 100L process to minimize the surprises at scale and provide a robust process. Your timeline and costs for development should include these activities.
QC Analytical Development
An obstacle of the QC Analytical Development of your LBP is the development of the bioburden assays, USP<61>/<62> which is not straightforward with a live organism. For LBPs these assays need to be tailored and developed for each organism and we have experience working on these assays. We are also able to harness a lot of efficiencies for the customer by not only working on the manufacturing but also working on the analytical development in-house. Analytical assays are necessary for both in-process testing and final QC testing for release.
Is List Labs the right CDMO for your project?
As a novice climber, would you attempt to scale Mt. Everest with anything less than the most experienced Sherpa? We are a passionate and dedicated team with the expertise and experience that is critical to reach the summit. We have been there many times. We recognize the obstacles and while no two ascents are exactly the same, we have the flexibility to guide you to the top and deliver a quality product for the success of your project. We look forward to working with you!
If you have any questions, please contact us at services@listlabs.com or through our contact page.
By: Stacy Burns-Guydish, Ph.D., President
Check out the Overcoming Obstacles in the development of Live Biotherapeutic Products video!
By: Rachel Berlin, Marketing Manager

List Labs is excited and proud to support microbiome research by providing reagents to scientists studying the human microbiome. Below is a list of microbiome research studies that have used List Labs’ research reagents such as Athrax, Pertussis, Cholera and Difficile Toxins.
Pertussis Toxins Used in Microbiome Research:
- Products #180 – Pertussis Toxin from B. pertussis, Lyophilized in Buffer and #181 – Pertussis Toxin from B. pertussis, Lyophilized (Salt-Free) have been used in the study of regulation of autoimmune myocarditis by host responses to the microbiome
- Product #179A – Pertussis Toxin from B. pertussis (in Glycerol) has been used in the study of bidirectional association between the gut micobiota and CNS disease in a biphasic murine model of multiple sclerosis
- Products #180 – Pertussis Toxin from B. pertussis, Lyophilized in Buffer and Product #181 – Pertussis Toxin from B. pertussis, Lyophilized (Salt-Free) were used in the study of CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice
- Products #180 – Pertussis Toxin from B. pertussis, Lyophilized in Buffer and #181 – Pertussis Toxin from B. pertussis, Lyophilized (Salt-Free) have been used to study the effect of omeprazole on the development of experimental autoimmune encephalomyelitis in C57BL/6J and SJL/J mice
- Products #180 – Pertussis Toxin from B. pertussis, Lyophilized in Buffer and #181 – Pertussis Toxin from B. pertussis, Lyophilized (Salt-Free) have been used to study an intestinal commensal symbiosis factor controls neuroinflammation via TLR2-medicated CD39 signalling
- Products #180 – Pertussis Toxin from B. pertussis, Lyophilized in Buffer and #181 – Pertussis Toxin from B. pertussis, Lyophilized (Salt-Free) have been used to show a commensal bacterial product elicits an modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation
- Products #180 – Pertussis Toxin from B. pertussis, Lyophilized in Buffer and #181 – Pertussis Toxin from B. pertussis, Lyophilized (Salt-Free) have been used to show dominant effects on the diet on the microbiome and the local and systemic immune response in mice
Difficile Toxins Used in Microbiome Research:
- Product #152C – Toxin A from Clostridium difficile and product #155 – Toxin B from Clostridium difficile were used in researching Immunogenicity and protective efficacy of recombinant Clostridium difficile flagellar protein FliC
- Product #152C – Toxin A from Clostridium difficile and product #155 – Toxin B from Clostridium difficile were used in researching an in vitro culture model to study the dynamics of colonic microbiota in Syrian golden hamsters and their susceptibility to infection with Clostridium difficile
Cholera Toxins Used in Microbiome Research:
- Product #103B Cholera Toxin B Subunit (Choleragenoid) from Vibrio cholerae and product #104 – Cholera Toxin B Subunit (Choleragenoid) from Vibrio cholerae in Low Salt were used to study the immunogenicty and effects on fecal microbiome of an electron-beam inactivated rhodococcus equi vaccine in neonatal foals
- Product #103B Cholera Toxin B Subunit (Choleragenoid) from Vibrio cholerae and product #104 – Cholera Toxin B Subunit (Choleragenoid) from Vibrio cholerae in Low Salt were used to study the effects of administration of live or inactivated virulent Rhodococcus equi and age on the fecal microbiome of neonatal foals
- Product #101 – Cholera Toxin from Vibrio cholerae was used in research finding that parental dietary fat intake alters offspring microbiome and immunity
- Product #171 – Anthrax Protective Antigen (PA), Recombinant from B. anthracis was used in research finding that substrate cleavage profiling suggests a distinct function of bacteroides fragilis metalloproteinases (fragilysin and metalloproteinase II) at the microbiome-inflammation-cancer inferface
In addition to producing products for microbiome research, List Labs also provides contract GMP manufacturing of live biotherapeutic products for phases 1-3 of clinical trials. For more information on microbiome and live biotherapeutics- check out this blog post or watch our informative video. See how scientists have used our products in their research on our citations page.
Contact us to discuss your next project!
By: Rachel Berlin, Marketing Manager
List Labs is proud to be exhibiting at the Microbiome Drug Develoment Summit 2018 in Boston, MA. The conference will be held at the Boston Seaport World Trade Center from June 20-22. This conference provides access to the latest preclinical, clinical and commercial case studies from the brightest minds in biopharma and academia to turn microbiome discoveries into patient therapies.
Come by our booth and ask us how we can help you develop materials for your clinical trials! Learn about List Labs’ services including Live Biotherapeutic Products. Check out how others have used our products in their microbiome research on our citations page.
Watch our informative video about our Microbiome and Live Biotherapeutic services.
Contact us to schedule a meeting with us at the show to discuss your next project!
By: Stacy Burns-Guydish, Ph.D., Senior Director, Production
The terms “Microbiome” and “live biotherapeutics” have been repeated frequently in the last few years in scientific circles. Researchers are understanding more and more about the various microbiomes in the human body and how they affect our overall health. From this research, microorganisms have been identified that may be beneficial to our health and could be used as a therapeutic, otherwise known as a live biotherapeutic products. This article explains an overview of some microbiome and live biotherapeutics basics.
What is Microbiome?
The human microbiome is the collection of trillions of microbes living in and on the human body. Scientists believe that it plays a role in many basic life processes and are important to our health. Perturbation of the microbiome has been associated with a growing number of diseases including inflammatory bowel disease, allergies, asthma, autism, and cancer.
What are some different types of human microbiomes?
Microbiomes are found, for example, in our gut, skin, vagina, and mouth. Each of these sites have a different consortia of microorganisms. Beneficial microorganisms have been identified in each of these niches. Researchers are studying the various human microbiomes to better understand their importance in health and disease.
What is a Live Biotherapeutic Product (LBP)?
A live biotherapeutic product contains a live microorganism that is used for the prevention, treatment, or cure of a disease or condition. As the characterization of the human microbiome and its link to human health has become better understood, microorganisms have been identified which may have a health benefit. The use of these microorganisms as a live biotherapeutic product in clinical application shows great promise. Several clinical trials are underway to evaluate their potential as a therapeutic.
What equipment and facilities are necessary for the production of a Live Biotherapeutic Products?
Many of the microorganisms identified for the manufacture of live biotherapeutic products are obligate or strict anaerobes and spore forming organisms. These type of organisms present unique challenges to the emerging microbiome therapeutic space. In particular, many of the microorganisms are anaerobes which cannot be exposed to air and thus expertise is required in handling and cultivating the organism. Facilities and equipment important for the cGMP manufacturing of these organisms include:
- Biological safety cabinets
- Anaerobic work stations with internal HEPA filtration for aseptic, anaerobic fill capability
- Equipment to scale the process cultivation
- Stainless steel or single use bioreactors
- Closed harvest systems such as tangential flow filtration with hollow fiber membranes
- Semi-automated or automated filling equipment
- Freeze drying equipment, i.e. Lyophilizer
- Homogenization and milling equipment
List Labs is your partner for Live Biotherapeutic Products
List Labs has manufactured several live biotherapeutic products for phase clinical trials. With 40 years of experience, List Labs is distinctly qualified to help you with your next microbiome project and affords the flexibility required to achieve the strictest timelines and goals. We understand that each project is unique and we draw from our vast experience to deliver you a custom solution that meets your needs. Contact us today to find out how we can help you!
By: Rachel Berlin, Marketing Manager
List Labs is proud to be exhibiting at the 4th Annual Translational Microbiome Conference April 18-20 at the Boston Marriott Long Wharf.

Interested in scheduling a meeting with us during the conference? Contact us!
By: Stacy Burns-Guydish, Ph.D., Senior Director, Microbiology
The human body is home to a vast ecosystem of microbes, called the microbiome. Research is shedding light on the importance of the microbiome as a benefactor to our health and development. Perturbation of the microbiome has been associated with a growing number of diseases including inflammatory bowel disease, allergies, asthma, autism, and cancer.
Microbiome Bacteria as Potential Therapy
Commensal bacteria of the microbiome are thought to be potential therapies for prevention or treatment of infections such as Clostridium difficile, acne, and bacterial vaginosis. Live biotherapeutic products (LBP) manufactured from commensal bacteria are being investigated by many companies and several clinical trials are underway.
Anaerobic Cultivation & Containment Required for Microbiome Research
Many of these commensal microorganisms for the manufacture of LBP are obligate or strict anaerobes and spore forming organisms, presenting unique challenges to the emerging microbiome therapeutic space. Expertise and proper equipment for anaerobic cultivation and proper containment of spore forming organisms is lacking in the industry and only a handful of contract manufacturing companies have the capabilities to perform GMP manufacturing anaerobic organisms, creating a bottleneck.
An alternative for companies is to build their own GMP facility, as Seres Therapeutics has done. But start-ups typically do not have the funds to build their own facilities thus the CMO backlog impacts timelines. Most CMO’s quote a wait of 9-12 months for projects to begin. So how will these burgeoning companies meet their aggressive timelines to produce their LBP for clinical trials?
Questions to Ask Partner GMP Labs
If you are a startup, sound advice is to start conversations now with potential CMOs and find out if their capabilities align with your organism cultivation requirements and specifications.
- How many Live Biotherapeutic Products have they produced and released?
- What is their experience with anaerobes?
- Can they work with spore formers?
- What are their QC and QA capabilities and regulatory support?
- What scale production is possible?
List Labs Is well suited to Partner in GMP Microbiome Research
List Biological Laboratories, a boutique contract manufacturing company, has the expertise and infrastructure for manufacturing both obligate and strict anaerobes and spore forming organisms. List has over 30 years’ experience cultivating anaerobic organisms. We have produced master and working cell banks and LBP for several customers currently in Phase 1 and 2 clinical trials. In addition, we have expertise in the development of the non-trivial required purity/bioburden assays, including USP61 and UPS62, for testing and release of these unique live microorganism products.
Contact List Labs to get your microbiome research project off the ground today.
By:
Debra Booth, VP of Operations
Linda Eaton, Ph.D., VP of Research & Development
Stacy Burns-Guydish, Ph.D., Senior Director of Microbiology
PJ Nehil, Sales & Distribution Coordinator
Dear Researchers Everywhere,
List Labs recently exhibited at the 2016 BIO International Convention. As a producer of bacterial products for research, as well as a provider of custom laboratory services, we were excited to meet with current and potential customers. We were eager to gain some insight into the current and future state of biotechnology and the up and coming field of microbiome research. But you never know exactly how you’ll feel until you spend the time in the conference exhibit hall. We were very pleased to see that we are a part of an industry that is moving forward at the pace of a start up, fueled by the novel ideas and intellect of many scientists.
Last week, thousands of people filled the Moscone Convention Center in San Francisco. The event was brilliantly organized and the venue was strategically arranged. The various pavilions were organized by state or country and some by specialty. It was a great opportunity to network and identify new sources for projects and services. Attendees were CEO and business development folks interested in learning more about what exhibitors have to provide. At our booth, we talked about immunotherapy, live biotherapeutics, contract manufacturing, GMP production, and more. It was a pleasure to shake hands with many customers, distributors, and colleagues and discuss ways we can partner with them to move their research forward.
We also had the opportunity to meet with vendors, in shipping, supplies and services. These vendors are critical to delivery of our biological and therapeutic products, and we benefited from learning about their new offerings as strategic partners. The enthusiasm was palpable from both exhibitors and attendees. At this event, we didn’t just meet industry veterans. We met many young scientists and job seekers looking for their first break. Some even came directly to us to hand-deliver their resumes. The event had a job expo, fueling another layer of energy and opportunity for exhibitors. We were resident in the California Pavilion where we learned that the State of California has a Biosciences Training Program, which will help companies pay for new employment training. Community colleges around the country are encouraging students to contribute to the future of biotechnology through clinical and regulatory apprenticeships. It’s great to see that science is providing opportunity for students in so many ways.
In closing, we found the 2016 BIO International Convention to be highly productive for our company. For those of you who didn’t get a chance to meet us at the convention, it’s very easy to reach us online and on social media. We would love to connect with you on LinkedIn, tweet with you on Twitter, like each other on Facebook and Google+. You can also check these accounts if you’re curious about your next opportunity to meet us at a conference. We even have a YouTube channel and a blog where you can learn more about us. We’d love to see your YouTube videos and read your blog if you have them as well. The future of biotechnology looks bright and we’re more excited than ever to be a part of it. We will definitely attend more events like this and we hope to see you at BIO 2017 in San Diego!
Regards,
Debra, Linda, Stacy, & PJ
By: Karen Crawford, Ph.D., President
Dear Microbiome Researchers,
I just returned from the Second Annual Translational Microbiome Conference in Boston and my head is spinning with the possibilities. Suggested connections between the microbial community living on/in our bodies and health are expanding from the health of our gut to asthma and beyond. Many in the field consider the Microbiome another organ, the most easily replaced or improved organ in the human body.
As we become increasingly aware that antibiotics both cure and create problems, it is encouraging to think that beneficial bacteria could be introduced and become a stable beneficial addition to our microbiome. Larry Weiss of AOBiome, a skin microbiome company, presented a product which can be obtained on the internet called Mother Dirt; a bottle with friendly bacteria originally derived from the soil, to spray on our bodies, replacing chemically-derived skin treatments such as soap and deodorant. Ammonia-utilizing bacteria in Mother Dirt convert naturally occurring nitrogen compounds on the skin to potentially beneficial nitrites.
Evolve Biosystems is looking at conditions which are rooted in perturbations in the microbiome of infants. An essential organism nicknamed “Baby Bif” is not present in high numbers in infants as a result of our modern aseptic, antibiotic filled environment. A skewed microbiome in infants may lead to conditions such as asthma/allergies, diabetes and obesity, conditions which could be prevented if friendly bacteria were provided in infant formula and foods. Laurel Lagenaur from Osel, Inc. presented data on lactobacillus products targeted to urinary tract and vaginal infections. Osel’s product, designed to restore a healthy vaginal microbiome, will likely be the first microbiome product to receive drug approval.
We heard about the OpenBiome stool bank which is providing materials for fecal transplants in multiple US centers. Success of the transplant procedures in resolving reoccurring C. difficile infections is fueling enthusiasm for development of pure culture therapies. Janssen Research and Development and Seres Therapeutics reported on projects to develop good gut bacteria as potential remedies for C.difficile, IBD, and Crohn’s disease. Janssen is using a network of collaborators to make progress in this area. Although the gut is the current focus, everyone is thinking beyond to using microbes to re-establish the balance of microbes to influence many different disease states.
Personalized nutrition was the headline from Lihi Segal of Day Two. The company is developing a personalized medicine approach to normalizing blood sugar. Feedback from a glucose monitor along with analysis of the gut microbiome allows Day Two to apply an algorithm suggesting meals to regulate blood sugar. Under such a regime the blood sugar roller coaster has been flattened for trial patients.
It is an exciting time to work in the microbiome arena, and I welcome the opportunity to connect with colleagues, meet up with customers and learn more about what others are doing to advance the study of the human microbiome. At List Labs, we pride ourselves on partnering to deliver live biotherapeutic products that yield results. If you are embarking on a new product or ready to identify a partner to ease into clinical trials with superior research, process development and manufacturing, contact us and find out more about how we collaborate.
Regards,
Karen
Dr. Karen Crawford, President of List Biological Laboratories, Inc.
We will be exhibiting at the 2nd Annual Translational Microbiome Conference at the Hilton Boston Back Bay on April 20-21. We are also proud to be a sponsor of this event.
If you will be in attendance, stop by the List Labs booth and chat with Karen Crawford about your research in the microbiome area. Karen is the president of List Labs and would enjoy speaking with you about our areas of expertise and how we would be able to support your microbiome or live biotherapeutic product work. We love hearing what companies are developing and finding ways to help.
If you want to learn more about our capabilities, check out our website or one of our videos and let us know how we can help you better position yourself to meet your research goals and objectives.
By: Suzanne Canada, Ph.D.
Tanager Medical Writing
While you go about your day, you are surrounded by micro-organisms. Although most of us spend a lot of time washing up and trying not to think about the propensity of creatures that share our personal space, scientists have been studying them. Due to their great progress, we are reaching an understanding of how these bacteria and fungi affect our bodies’ functions [1]. The evidence indicates that this inner-ecosystem can not only cause disease if perturbed, but also influence our overall health! Organisms including Escherichia coli, Helicobacter pylori, Streptococcus thermophilus, and species of Clostridia, Lactobacillus, and Bacterioides inhabit our gut. Corynebacterium jeikeium as well as Staphylococcus species live on our skin, and other Streptococci as well as Neisseria and Candida albicans inhabit our mouth and upper respiratory system [2]. The makeup and diversity of organisms has been found to be strongly influenced, not only by what you eat [3], but also by who you live with [4, 5]. With greater understanding of this rich soup of life that we carry with us, the microbiome has become the new frontier in cutting-edge drug development [6].
In the last three years, research into the molecular basis of microbial influence has blossomed. The first and most obvious application for this information was in treating C. difficile infections; which result from overgrowth of the opportunistic pathogen after an antibiotic regimen or hospital stay. Researchers found that fecal transplants from a healthy individual were an effective way to treat this potentially fatal infection [7, 8]. The role of intestinal microbes in Inflammatory Bowel Disease (IBD) has been established [9] in the last year or two. Based on this knowledge, possible treatments for IBD, such as ulcerative colitis and Crohn’s disease, are in development. Other publications point to microbes’ role in inflammation of the skin and respiratory tract, including acne [10]and asthma[11]. More excitement has been generated as investigators have found links to other chronic diseases including diabetes [12, 13], hypertension [14, 15], and chronic liver disease [16]. Preliminary investigations suggest a connection between overall gut microbial composition and obesity [17]. Some studies in mouse models have even linked the microbiome to the neurological conditions of Alzheimer’s [18] and autism [19, 20].
With all this research going on, you need a great resource like LIST Biological Laboratories, with experience and expertise with microbial products spanning over 25 years. LIST has several products available that can serve as positive controls for your microbial research. Potent toxins from C. difficile are available (LIST products #157, #158), as well as antibodies that aid in their detection (LIST products #753, #754). Lipopolysaccharides are also available, which cause inflammation and activation of immune signaling cascades, and are extracted from bacterial cell walls of E. coli O111:B4, O55:B5, O157:H7, J5 and K12; Salmonella typhimurium, Salmonella minnesota and Bordetella pertussis. Other acute immune system activators such as Staphylococcal toxins (LIST products #120, #122) and Shiga toxins (LIST products #161 & #162) are also available.
In case the assortment of purified bacterial products on hand are insufficient for your research needs, LIST also provides contract manufacturing for biotherapeutics, as well as microbial purification services.
References
- Human Microbiome Project, C., A framework for human microbiome research. Nature, 2012. 486(7402): p. 215-21. PMID: 22699610
- Human Microbiome Project, C., Structure, function and diversity of the healthy human microbiome. Nature, 2012. 486(7402): p. 207-14. PMID: 22699609
- David, L.A., et al., Diet rapidly and reproducibly alters the human gut microbiome. Nature, 2014. 505(7484): p. 559-63. PMID: 24336217
- Yatsunenko, T., et al., Human gut microbiome viewed across age and geography. Nature, 2012. 486(7402): p. 222-7. PMID: 22699611
- La Rosa, P.S., et al., Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A, 2014. 111(34): p. 12522-7. PMID: 25114261
- Donia, M.S., et al., A Systematic Analysis of Biosynthetic Gene Clusters in the Human Microbiome Reveals a Common Family of Antibiotics. Cell, 2014. 158(6) p1402 – 1414. PMID: 25215495
- Seekatz, A.M., et al., Recovery of the gut microbiome following fecal microbiota transplantation. MBio, 2014. 5(3): p. e00893-14. PMID: 24939885
- Scott, K.P., et al., Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis, 2015. 26: p. 25877. PMID: 25651995
- Huttenhower, C., A.D. Kostic, and R.J. Xavier, Inflammatory bowel disease as a model for translating the microbiome. Immunity, 2014. 40(6): p. 843-54. PMID: 24950204
- Christensen, G.J. and H. Bruggemann, Bacterial skin commensals and their role as host guardians. Benef Microbes, 2014. 5(2): p. 201-15. PMID: 24322878
- Martin, C., et al., Host-microbe interactions in distal airways: relevance to chronic airway diseases. Eur Respir Rev, 2015. 24(135): p. 78-91. PMID: 25726559
- Tang, D., et al., Comparative investigation of in vitro biotransformation of 14 components in Ginkgo biloba extract in normal, diabetes and diabetic nephropathy rat intestinal bacteria matrix. J Pharm Biomed Anal, 2014. 100: p. 1-10. PMID: 25117949
- Sato, J., et al., Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care, 2014. 37(8): p. 2343-50. PMID: 24824547
- Pluznick, J., A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes, 2014. 5(2): p. 202-7. PMID: 24429443
- Pluznick, J.L., Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol, 2013. 305(4): p. F439-44. PMID: 23761671
- Minemura, M. and Y. Shimizu, Gut microbiota and liver diseases. World J Gastroenterol, 2015. 21(6): p. 1691-702. PMID: 25684933
- Al-Ghalith, G.A., P. Vangay, and D. Knights, The guts of obesity: progress and challenges in linking gut microbes to obesity. Discov Med, 2015. 19(103): p. 81-8. PMID: 25725222
- Bibi, F., et al., Link between chronic bacterial inflammation and Alzheimer disease. CNS Neurol Disord Drug Targets, 2014. 13(7): p. 1140-7. PMID: 25230225
- De Angelis, M., et al., Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One, 2013. 8(10): p. e76993. PMID: 24130822
- Pequegnat, B., et al., A vaccine and diagnostic target for Clostridium bolteae, an autism-associated bacterium. Vaccine, 2013. 31(26): p. 2787-90. PMID: 23602537