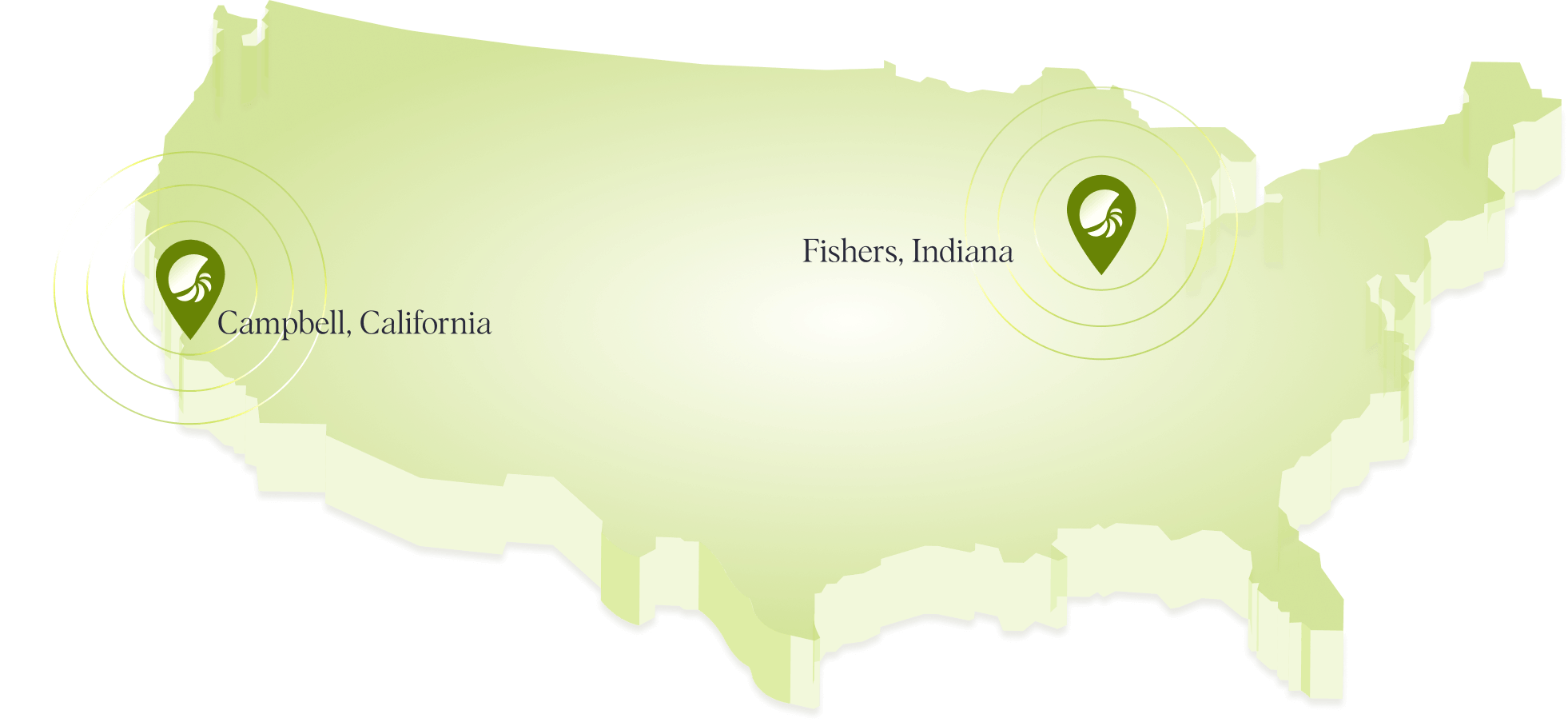
List Labs is proud to announce the upcoming launch of our facility in Fishers, Indiana. Spanning over 120,000 square feet, this state-of-the-art cGMP compliant manufacturing facility designed to meet global standards is slated to go online in Q4 2024.
The multi-product facility represents a major step forward in our commitment to delivering a comprehensive end-to-end solution, including scalable capabilities for: